the model food code is a federal law enforced by the fda
All About the FDAs Food Code. The United States Food Code is a model created by the Food and Drug Administration FDA for the purpose of regulating any entity that sells manufactures or provides food as part of their services.

Digital Health Report 2022 Usa
The gov means its official.

. This law was instituted because food. The FDA Food Code is not federal law. The word law when used in the Food Code really means any applicable local state and federal statute regulation or ordinance.
The model Food Code is neither federal law nor federal regulation and is not preemptive. Laws may have been amended since original date Federal Food and Drugs Act of 1906 repealed. Food code is a reference document for regulatory agencies responsible for overseeing food safety in retail outlets such as restaurants grocery stores and institutions.
A model food code for jurisdictions to adopt B. FDA 2017 Model Food Code is a model code that assists food safety jurisdictions at all levels of government by providing them with a scientifically. The updates to the Food Code amended the minimal cooking time in seconds for comminuted meat from 15 seconds to ____ seconds.
Rather it represents FDAs best advice for a uniform system of regulation to ensure that food at retail is safe and properly protected and presented. Food and Drug Administration Amendments Act of 2007 PL 110-85 Sept. Also what is the FDA Code of Federal.
Applicable local state and federal statutes regulations and ordinances. Before sharing sensitive information make sure youre on a federal government site. The FDA Food Code is a model code guideline that provides over 3000 local state tribal and federal food control agencies scientifically sound food safety information that follows national food regulatory policies.
Rather it represents FDAs best advice for a uniform system of regulation. An FDA law C. It represents FDAs best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service.
The Conference for Food Protection CFP sends its recommendations for. Food code is neither a federal law or regulation rather it is the FDA best advice for. Also called the Food Drug and Cosmetic Act.
The FDA Food Code is A. The Food Code is published in its FULL version every ____ years. Dates shown are when Public Law was first approved.
Rather it represents FDAs best advice for a uniform system of regulation. It represents FDAs best advice for a uniform system. 21 CFR Parts 1-1299 is a federal law relating to food other than USDA-regulated products enforced by the FDA.
Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment of the industry restaurants and grocery stores and institutions such as nursing homes. The model Food Code is neither federal law nor federal regulation and is not preemptive. The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer.
Federal requirements until adopted by federal bodies for use within federal jurisdictions the model Food Code provisions are designed to be consistent with federal food laws and regulations and are written for ease of legal adoption at all levels of government. The Food Code originated from the Pure Drug and Food Act of 1906. Its been amended over 100 times.
The latest amendment known as the Food Safety Modernization Act is the most significant amendment of US. A list of jurisdictions that have reported to FDA their status in adopting. It is the FDAs best advice for ways to ensure that food at retail and in foodservice is safe properly protected and presented.
A state law 2. A Food Code section number such as 3-40310 denotes. Local state tribal and federal regulators use the FDA Food Code as a model to develop or update their own food safety rules and to be.
The model Food Code is neither federal law nor federal regulation and is not preemptive. Federal government websites often end in gov or mil. The model Food Code is neither federal law nor federal regulation and is not preemptive.
27 2007 More information about FDAAA Other Laws Affecting FDA. Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment of the industry restaurants and grocery stores and. The FDA Food Code is a model code guideline that provides over 3000 local state tribal and federal food control agencies scientifically sound food safety information that follows national food regulatory policies.
Food law in history. Rather it represents FDAs best advice for a uniform system of regulation. This law as amended is still in force today.
The Food Code is guidance representing FDAs current thinking and is a model on safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. The FDA Food Code is not federal law. Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the.
FDA Food Safety Regulations and Model Codes from Farm to Fork - -Produce Safety Rule.
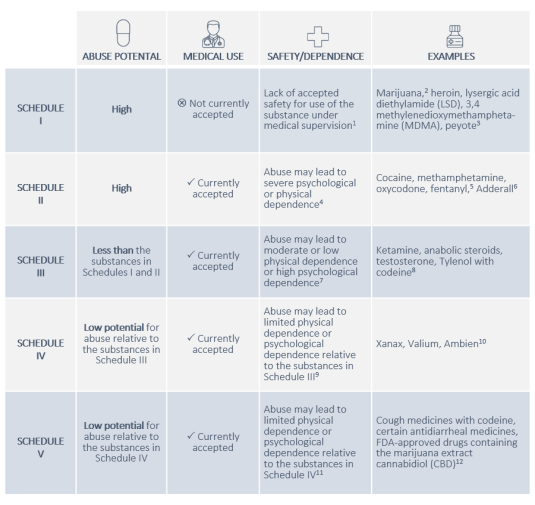
The Controlled Substances Act Csa A Legal Overview For The 117th Congress Everycrsreport Com
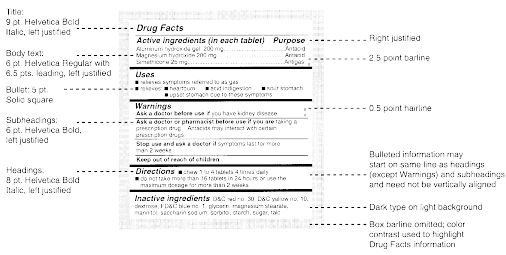
Cfr Code Of Federal Regulations Title 21

Is It Time For The Fda To Become An Independent Body Stat

Crickets Pot And Pigs Oh My A View Of 2022 Institute For Food Laws And Regulations

The Case For A Federal Robotics Commission

Federal Hazardous Substances Act Fhsa Requirements Cpsc Gov
.png.aspx)
The Importance Of The Device Label To A Global Udi System Raps

Cfr Title 21 Pt 170 199 Code Of Federal Regulations 2021 U S Government Bookstore

Pharmaceutical Advertising Report 2021 2022 Usa

Key Questions About Covid 19 Vaccine Mandates Kff
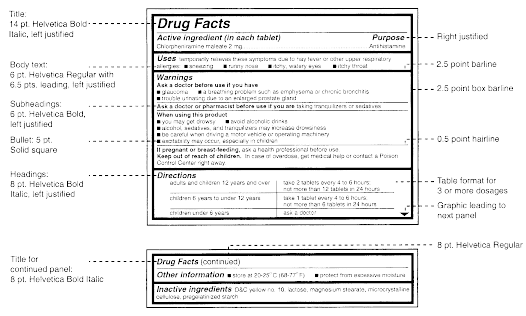
Cfr Code Of Federal Regulations Title 21
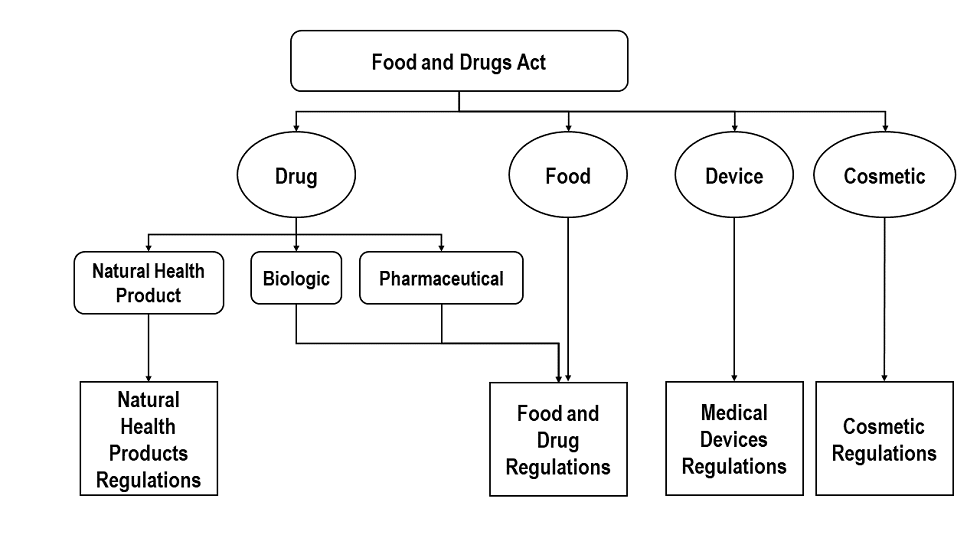
Classification Of Products Under The Food And Drugs Act F Da Canada Ca

Guidance Document Part C Division 5 Of The Food And Drug Regulations Drugs For Clinical Trials Involving Human Subjects Gui 0100 Canada Ca

The Difference Between Fda Registered Fda Approved And Fda Cleared Aspen Laser
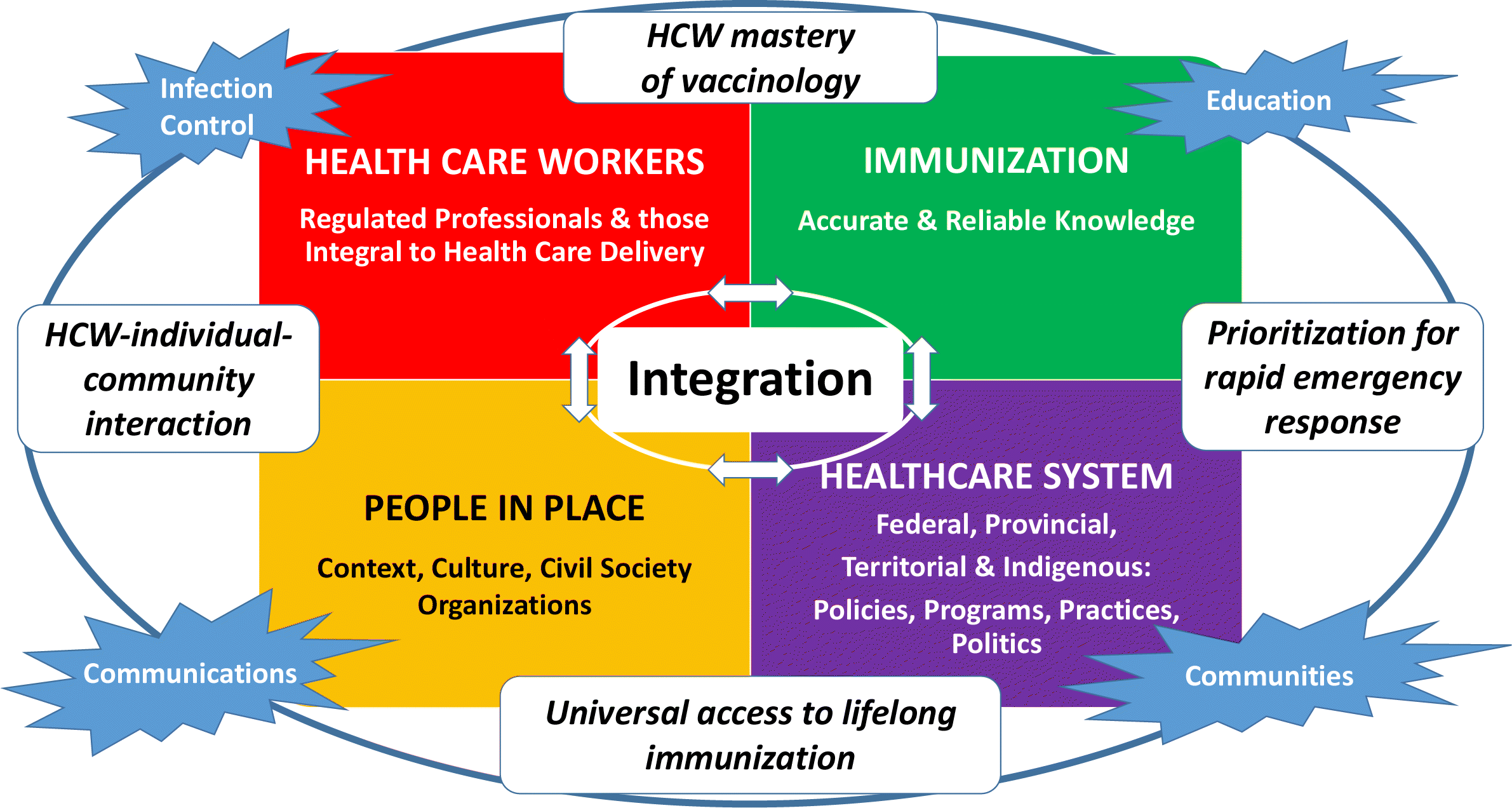
Royal Society Of Canada Covid 19 Report Enhancing Covid 19 Vaccine Acceptance In Canada
